Granular cast
AKI Evaluation
The high prevalence of acute kidney injury (AKI) in hospitals makes it imperative to have, in hand, a systematic method for management of this common malady. The number of ways a kidney can malfunction is intimidating, but when looking at a patient with AKI, a fairly simple series of questions can lead one to the correct diagnosis with apparent deft clinical ability.
Define AKI
To begin, diagnose the presence of AKI. Do not mince your language. Avoid being the one who incorrectly labels someone has having AKI based soley on a rising BUN after glucocorticoid initiation. At it’s core, AKI is present with the serum Cr is 1.5-1.9x baseline over the course of 1 week or when serum Cr rises at least 0.3mg/dL over 48h. The definition of AKI can also be made when urine output (UOP) is <0.5mL/kg/hr for 6-12h. There are stages of AKI which also exist (stage 1-3 indicative of rising severity of AKI). These are largely used for research purposes and are not terribly useful in clinical practice. If you’re patient develops AKI, you need to figure out why it’s the case and reverse the cause to improve her outcome.
Before we go further, one may ask why acute kidney injury is important. Of course, hyperkalemia or pulmonary edema with AKI lead to patient deterioration, but there are general mortality risks associated with AKI. For example, a serum Cr rise of 0.3mg/dL in 48h is associated with increased mortality. Mortality risk increases with also increasing AKI stage of severity. The odds ratio for each stage of AKI in hospitalized are as follows. Stage I (OR 2.2), stage II (OR 6.1), stage III (OR 8.6). The mortality risk of AKI was shown to be ~45% on one study (all previous data is from from Murugan 2011).
Find the cause
The next step is to find the reason for AKI. If your patient meets criteria for AKI, then you already know that they’re risk of dying is higher. Now is the time to identify the cause of AKI, treat the cause, be the hero, and save a life. There are approximately about 1001 ways for a kidney to break. During the course of a hospital work day, meticulously going through hundreds of possibilities will clearly not work. Sure, you could just blame it on prerenal azotemia from true volume depletion, but how do you know for sure? The following method is a simpler way to go about finding the correct diagnosis. By thinking about the most common causes of AKI as well as having high sensitivity, but low specificity triggers for considering rare diagnoses (i.e. bilateral occlusion of both renal arteries), one can move through the causes of renal failure with speed and high accuracy. This is when nephrology becomes fun.
A reduction in cognitive load during the diagnosis process is useful. Focusing on the pertinent data is what separates 3rd year medical students from upper level residents. Too many things on the mind is what makes a developing clinician in a short white coat spend minutes talking about a potassium of 5.2 mmol/L, but forget to mention the fact that the patient’s blood cultures turned positive overnight.
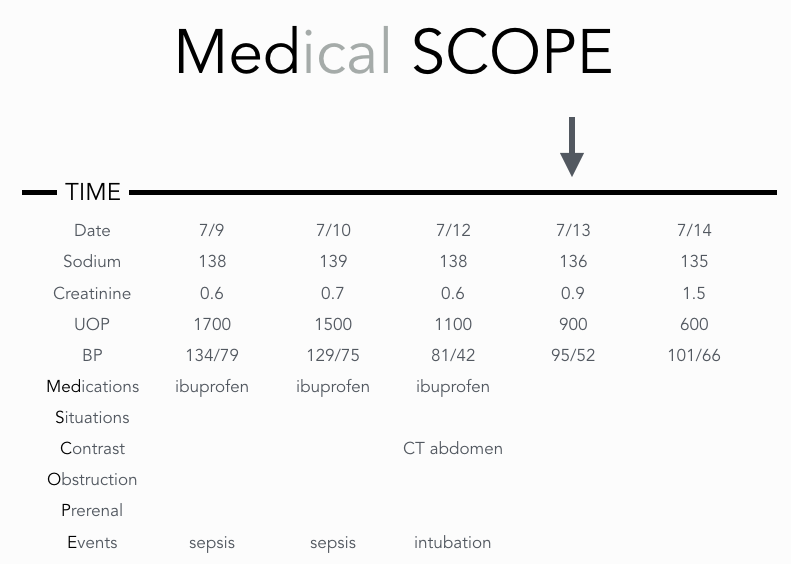
Is it even possible to remember every kind of renal failure in a few words? Yes. To do so, use the acronyms “SCrUB” and “Medical SCOPE.” After you have diagnosed the presence of AKI, SCrUB the patient’s chart for standard numbers that you should know. Trend the numbers for serum Sodium, serum Creatinine, Urine output, and Blood pressure. These are the mints on the hotel pillow that you should come to expect as standard. The end product will look like this…
The first thing we see is that the serum creatinine increased on 7/13 (arrow) and continued to worsen the next day. Bingo, we have a smaller timeframe of events that could have caused AKI. The first thing to note is that the patient is in the hospital. Is it possible that they developed anti-GBM disease during their stay for acute appendicitis? Although it would feel pretty cool to make this diagnosis in this setting, it is simply not going to be the cause of AKI. It’s more likely that the culprit is something that we did to the patient that harmed him or her. Lining up all these numbers allows us to see important trends. Why look at serum sodium? If it’s elevated, start thinking prerenal azotemia from true volume depletion unless you have another explanation for hypernatremia. Why care about UOP? If the UOP was 1.5L a day until a foley was removed and you observe the UOP falling to zero, then think urinary obstruction. Finally, look at blood pressure. A patient on 3 pressors can be expected to have acute tubular injury (ATI). Occasionally though, a patient will have poorly controlled blood pressure at home, come into the hospital at which point, the blood pressure is reduced to 120/80 by an overzealous individual. ATI can actually happen in situations like this and it is not uncommon. In these situations, spin the urine to find granular casts and arrive at a diagnosis of normotensive acute tubular injury. The purpose of “SCRUB” is to align your thinking with the most likely cause of renal failure. It will prevent you from being the one suggesting glomerulonephritis as a top differential in this case. “SCRUBbing the chart” for these numbers will prevent you from missing the obvious cause of AKI
And now, we move onto the finer points of AKI diagnosis. It doesn’t take much sleuthing to diagnose ATI as the cause of renal failure in a patient with gram negative sepsis on 3 pressors. The reason nephrologists exist is to also exclude the possibility of rare disorders so that patient care can continued in an ordered and efficient manner without ordering needlessly excessive testing. How is this done? Should one shoot from the hip and order thousands of dollars of serological tests on every AKI (like ANCA, anti-GBM ab, etc)? Of course this is wasteful and simple-minded.
Use the acronym “MEDical SCOPE” to sift through potential rare diagnoses. The purpose of “MEDical SCOPE” is to have a series of high sensitivity, but low specificity triggers for considering a particular diagnosis. It is somewhat the review of systems for renal failure. It’s an efficient way of building a differential diagnosis from which you can pursue more formal evaluation. It prevents you from taking a lazy diagnosis of ATI, when another disorder is actually occurring. This thought process is something that makes you a good clinician. It the thought process that will allow you to make the occasional rare diagnosis.
MEDical SCOPE first evaluates MEDications for cause fo reanal failure. It then goes on to look at Situations that are commonly associated with renal failure. Patients admitted for pulmonary edema from a heart failure exacerbation could reasonably have cardiorenal syndrome. Other examples are listed below. Contrast administration is then examined. Intra-arterial contrast causes renal failure. Intravenous iodinated contrast is something every nephrologist has seen countless times. Multiple studies question the existence of contrast-induced nephropathy though. Keep current on the evolving literature on this subject. Gadolinium contrast does not cause acute kidney injury, but can cause nephrogenic systemic fibrosis when used in renal failure. A last note is that while most angiography uses iodinated contrast, make sure to read the procedure note as carbon dioxide is sometimes used as contrast. You don’t want to be the one who blames AKI on carbon dioxide. Obstruction includes not only protate issues, but neurogenic bladders and clots from gross hematuria. Remember that patients with normal renal function have renal reserve so that if they get a unilateral obstructing renal stone, no change in serum Cr will occur. However, patients with CKD 3 and worse have lost their renal reserve and a unilateral obstructing renal stone can cause AKI. Prerenal azotemia from true volume depletion is usually easy to spot or diagnose after a night of IV fluid administration. Look for orthostatic vital signs, decreased skin turgor under the clavicles, and sunken eyes. Also remember that hypercalcemia acts like a combination of a loop diuretic and tolvaptan. Patients with severe hypercalcemia will be volume depleted. Certain Events, as shown on the chart, are often associated with AKI.
Medications commonly associated with renal failure:
NSAIDs: renal failure from NSAIDs can be caused by all NSAIDs including COX-2 inhibitors (Perazella 2001) and aspirin (Berg 1977). Renal failure due to NSAIDs is most likely to happen in a volume-depleted state.
ACE inhibitor and angiotensin receptor blockers: can increase the risk for AKI when combined with other factors leading to AKI.
Amphotericin B (liposomal formulation): Causes AKI ~15% of the time (Mistro 2012). The onset of AKI typically occurs in 5-9 days after starting treatment (Karimzadeh 2016).
AIN: there are many medications associated with AIN. Only 5-10% of patients present with the classic triad of fever, rash, and eosinophilia. Fever is notably absent in NSAID-induced AIN, but is present in 50-100% of patients with AIN attributed to penicillin derivatives. Across all classes of drugs, fever is present in 30%. Rash is present in 15-50%. Eosinophilia occurs in 80% of cases of AIN from beta-lactams, but is present in no more than one-third of cases caused by other medications. Leukocytes are present in nearly all cases of AIN due to methicillin, but are noted in less than half of cases of AIN due to other medications (Perazella 2010). Eosinophiluria has a sensitivity of 31% and a specificity of 68% in biopsy-proven acute interstitial nephritis (Muriithi 2013). Findings of WBC casts in urine sediment without pyelonephritis is highly suggestive of AIN (Perazella 2010).
Situations Associated with Renal Failure
Actue heart failure exacerbation: is cardiorenal syndrome present?
Cholesterol Emboli: associated with manipulation of arteries. 75% have skin findings. 66% of eosinophilia (>500cells/uL). Most have subacute presentation with onset of AKI 2-6 weeks after event. 20% have an acute presentation with onset within 1 week after event (Scolari 2007).
Tumor lysis syndrome
Decompensated cirrhosis: is hepatorenal syndrome present? The international Club of Ascites criteria defines HRS when the following criteria are met (Angeli 2015):
There is a diagnosis of ascites
There is a diagnosis of AKI according to ICA-AKI Criteria
No response after 2 consecutive days of diuretic withdrawal and plasma volume expansion with albumin 1g/kg body weight
There is an absence of shock
No current or recent use of nephrotoxic drugs or contrast
No macroscopic signs of structural kidney injury (defined as absence of >500mg/day proteinuria, absence of >50 RBCs/hpf, normal renal ultrasound findings)
If you place a foley in a patient who isn’t on 4 pressors and the foley is 100% dry, then the differential diagnosis narrows to acute occlusion of renal arteries or bilateral urinary obstruction above the level of the prostate (as in retroperitoneal fibrosis).
Thrombocytopenia: if AKI occurs in the setting of thrombocytopenia of unknown etiology, then thrombotic microangiopathy (due to atypical HUS, TTP, HUS, or TMA from other cause) rises on the differential diagnosis. Order urgent workup for microangiopathic hemolytic anemia (MAHA) to see if urgent plasmapheresis is needed.
Hemoptysis: if renal failure and hemoptysis are present, then a pulmonary-renal syndrome should be at the top of the differential diagnosis (Goodpasture disease, ANCA vasculitis, lupus nephritis, other vasculitis).
Hypernatremia: if AKI is present in the setting of any degree of hypernatremia, then prerenal azotemia from true volume depletion is likely present.
Hypercalcemia, unexplained anemia, lytic lesions: if these are present, consider multiple myeloma.
Recent surgery: look at anesthesia notes for intraoperative hypotension
Contrast
The onset of AKI is typically 24-48h after contrast exposure, but note that the actual existance of contrast-induced nephropathy has come into question. Keep up-to-date on the literature for this topic as it will be interesting. Everyone in the hospital “sees” “contrast-induced nephropathy” all the time, but actual studies make it questionable if CIN exists at all.
Iodinated contrast can cause AKI. Gadolinium-based contrast does not cause AKI, but can cause nephrogenic systemic fibrosis.
Obstruction
Consider obstruction more strongly in older men, those with recent surgery, AKI in patients with lower midline abdominal pain (from distended bladder), gross hematuria with clots, recent foley removal.
Prerenal Causes
Consider in the proper setting. The following physical findings are are associated with the following likelihood ratios for volume depletion being present or absent: dry axilla (LR 3.0/LR 0.6), dry mucous membranes of mouth and nose (LR 3.1/LR 0.4), sunken eyes (LR 3.7LR 3.7), decreased skin turgor in subclavicular area (LR 3.5LR 0.3). *Information from Evidence-based Physical Diagnosis, 4e, Steven McGee
Events
The following events are all associated with AKI from various causes: cardiac arrest, surgery, hypotension after intubation, causes of rhabdomyolysis (seizures, influenza, cocaine, trauma, extreme exertion, malignant hyperthermia, neuroleptic malignant syndrome, amphetamines, other medications), large volume paracentesis.
Testing
What testing should be ordered? Get a urinalysis on all patients and spin the urine yourself… on every patient. If you know the diagnosis, don’t get a renal ultrasound. If you are unsure… get one. A renal ultrasound poses no risk to the patient and it is simply unacceptable to miss a diagnosis of hydronephrosis. If you consult nephrology, by definition, you are almost by definition unsure of the diagnosis or renal failure is severe and so you should order one a renal ultrasound. If there is any suspicion of rhabdo, add a CPK onto the renal chem10. Almost never worry about getting urinary electrolytes or FENa unless it is obtained before IV fluids are given in the ED. We infrequently place a large importance on urine electrolytes. Even when considered, they are viewed in the context of the big picture. They are very much less important than history and an overnight response to isotonic crystalloids. Never get urine eosinophils. Don’t bother getting 24h urine collections — we would only consider getting this if we are planning a kidney biopsy or the patient likely has a nephrotic or nephritic syndrome. Don’t order glomerular testing unless you are a nephrologist and/or have an incredibly good reason to do so. It takes a reasonably high level of suspicion to order serological testing appropriately. Nephrologists and seasoned clinicians are good at sniffing out glomerular disorders, but if you are in training, it is best to double check these orders with an attending first. .
Start treatment
Start treatment. If you think true volume depletion is present, start isotonic crystalloids. A good place to start with volume replacement is this — there are only 3 isotonic fluid rates that matter — 50mL/hr, 75mL/hr, and 125mL/hr. A normal rate is 75mL/hr. If you are admitting a patient late at night, you may want to use a rate of 125mL/hr if there is no concern for pulmonary edema or some other abnormality like hyponatremia. If you are taking care of a patient and you want to give IV fluids, but they have a poor functional status, or you are worried about causing volume overload, consider a rate of 50mL/hr. Think about obstruction. Get a bladder scan and place a foley if the PVR is >300mL or if the patient has suprapubic pain and cannot void. Alternatively, if the renal ultrasound has already resulted and shows no hydronephrosis, you can forego this. Even better, use point-of-care ultrasound to assess for this at the bedside. If there is severe AKI (Cr >3x baseline), severe fluid overload (as in you are at the bedside placing orders for a STAT CXR, ABG, ECG, troponin), lack of hypotension, concern for need of dialysis and poor UOP and there is pulmonary edema (requiring 35-50% venti-mask), don’t be shy with lasix. Give a minimum of 80-120mg IV lasix. Place a foley STAT. If the patient does not make 100-200mL/hr UOP within the first hour, then double the dose. If the second dose does not work, then be thinking that urgent dialysis may be what is needed. Redose medications that need to be reduced (cefepime; vanc; opiodids; gabapentin).
The last question is if dialysis is needed. A simple mnemonic AEIOU is used to remember the reasons for this… acidosis, electrolyte abnormalities, ingestion (poisonings), fluid overload, and uremia. Dialysis utility in poisonings won’t be addressed here, but trigger points for thinking about the need for urgent dialysis can be derived from a 2016 NEJM article (Gaundry 2006) examining the effectiveness of early vs. late renal replacement therapy initiation. These patients had a diagnosis of stage 3 AKI due to acute tubular injury and were on mechanical ventilation, pressors, or both. Indications for renal replacement therapy assigned to the delayed strategy of renal replacement therapy initiation were:
BUN >112mg/dL
Serum K+ >6.0mmol/L (or greater than 5.5mmol/L despite medical treatment)
a pH below 7.15 in the context of either pure metabolic acidosis or mixed acidosis (Paco2 at least 50 mm Hg) without the possibility of increasing alveolar ventilation
Acute pulmonary edema due to fluid overload responsible for severe hypoxemia requiring an oxygen flow rate >5L/min to maintain SaO2 >95% or those on mechanical ventilation with FiO2 >50% despite diuretic therapy
Every patient is different, but these are solid indications for dialysis that most nephrologists would agree with, other than the fact that you would really need to have a manifestation of uremia to dialyze someone with a high BUN. You are now armed with the knowledge to diagnose almost any type of AKI and get them through the night for further evaluation and treatment.
REFERENCES
Angeli, P., Ginès, P., Wong, F., Bernardi, M., Boyer, T. D., Gerbes, A., ... & Moore, K. (2015). Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut, 64(4), 531-537.
Berg, K. J. (1977). Acute effects of acetylsalicylic acid in patients with chronic renal insufficiency. European journal of clinical pharmacology, 11(2), 111-116.
Garrick, R. (2008). The Challenge of Diagnosing Atheroembolic Renal Disease: Clinical Features and Prognostic Factors Scolari F, Ravani P, Gaggi R, et al (Univ and Spedali Civili, Brescia, Italy; Mem Univ of Newfoundland, Canada; Ospedale Malpighi, Bologna, Italy; et al) Circulation 116: 298-304, 2007. Year Book of Medicine, 2008, 240-241.
Gaudry, S., Hajage, D., Schortgen, F., Martin-Lefevre, L., Pons, B., Boulet, E., ... & De Prost, N. (2016). Initiation strategies for renal-replacement therapy in the intensive care unit. New England Journal of Medicine, 375(2), 122-133.
Karimzadeh, I., Heydari, M., Ramzi, M., & Sagheb, M. M. (2016). Frequency and Associated Factors of Amphotericin B Nephrotoxicity in Hospitalized Patients in Hematology-Oncology Wards in the Southwest of Iran. Nephro-urology monthly, 8(5).
McGee, S. (2012). Evidence-based physical diagnosis e-book. Elsevier Health Sciences.
Mistro, S., Maciel, I. D. M., de Menezes, R. G., Maia, Z. P., Schooley, R. T., & Badaró, R. (2012). Does lipid emulsion reduce amphotericin B nephrotoxicity? A systematic review and meta-analysis. Clinical infectious diseases, 54(12), 1774-1777.
Muriithi, A. K., Nasr, S. H., & Leung, N. (2013). Utility of urine eosinophils in the diagnosis of acute interstitial nephritis. Clinical Journal of the American Society of Nephrology, 8(11), 1857-1862.
Murugan, R., & Kellum, J. A. (2011). Acute kidney injury: what's the prognosis?. Nature Reviews Nephrology, 7(4), 209.
Perazella, M. A., & Tray, K. (2001). Selective cyclooxygenase-2 inhibitors: a pattern of nephrotoxicity similar to traditional nonsteroidal anti-inflammatory drugs. The American journal of medicine, 111(1), 64-67.
Perazella, M. A., & Markowitz, G. S. (2010). Drug-induced acute interstitial nephritis. Nature Reviews Nephrology, 6(8), 461.